When we use GC-MS for forensic science purposes for controlled substance detection, we can basically divide the world into two camps. First, is the world of solid drug dose in pre-consumption form. The second is in the world post-consumption form analysis in a biological matrix (blood, urine, or oral fluid) which is otherwise known as toxicology.
Is this white stuff cocaine or is it a crushed up Tylenol PM? This is the job of the analyst who does solid drug dose in pre-consumption form testing.
Did the arrested driver have any controlled substance in his blood? This is the job of the analyst who does toxicology.
The GC-MS is used differently in these two contexts.
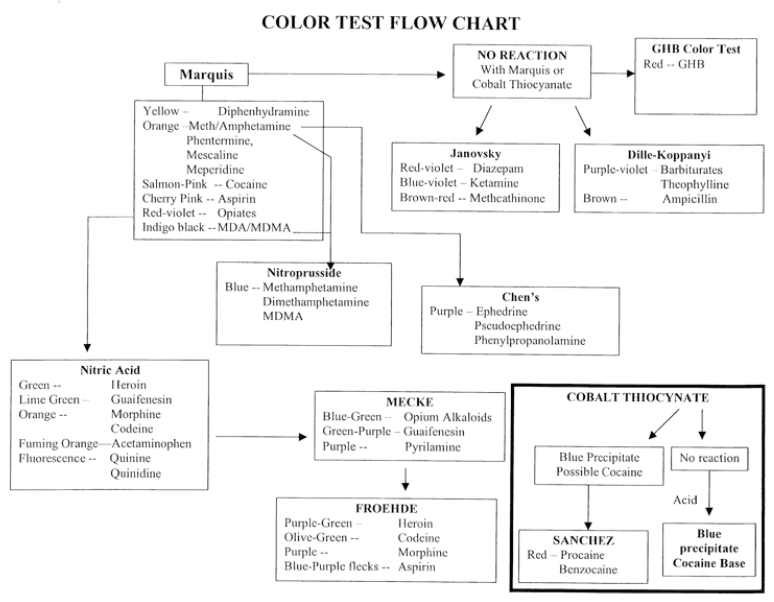
In the solid drug dose in pre-consumption form testing the suspected drug is typically tested in the laboratory by way of a series of screening colormetric tests, and then by single quadrupole GC-MS in a specific way.

 In the subsequent GC-MS testing during the pre-consumption form analysis, a separate sample is extracted and typically placed into a solvent with the result being direct liquid injected into the GC-MS for analysis with the single quadrupole analysis set at the scan mode.
In the subsequent GC-MS testing during the pre-consumption form analysis, a separate sample is extracted and typically placed into a solvent with the result being direct liquid injected into the GC-MS for analysis with the single quadrupole analysis set at the scan mode.
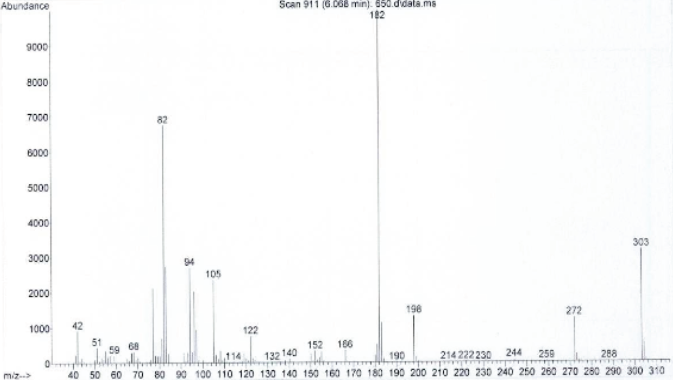
- A spectrum derived by setting the GC-MS mode at scan
As you can see from the above, when we do it this way (scan mode), we get a lot of fragmentation typically. The greater the fragmentation, the richer the data (information) resulting in a lot of ions. This rich information allows us to interpret the spectrum and make a qualitative call. The more information, the lower the risk of being wrong in our qualitative identification. The price of this greater information and the lowering of the risk of being wrong is that we lose our quantitative strength.
On the other hand, in the world of post-consumption form testing in a biological matrix (blood, urine, or oral fluid) which is otherwise known as toxicology, the same instrument is ultimately used (referring to the GC-MS), we do not set it to scan. We set it to Selective Ion Monitoring (SIM). With the SIM setting, we select for specific ions. For example, some laboratories use m/z 371, m/z 473 and m/z 488 for marijuana. This means that the laboratory has set the GC-MS to only detect at those specific ions and those ions alone. It is blind to any other ions that may be present. It is only looking for these three ions and these three alone. They are called the diagnostic ions. By setting the GC-MS to SIM mode we lose all of that beautiful fragmentation data and only settle for the information present for our diagnostic ions. See the below comparison in scan versus SIM of the same unknown:

By switching to SIM, we are able to quantitate much more reproducibly and with lower uncertainty in our measurement.
But here is my question, other than “experience” or “this is the way we always do it,” how do we determine those three chosen diagnostic ions are truly unique to the analyte of interest so as to qualify as a specific qualitative measurement? I have looked at a lot of what is passed off as validation studies. A lot of what I see is basically intermediate precision studies that are analysis of replicates. It seems to me that there are a lot of open questions when toxicological samples are run when it comes to the qualitative interpretation in these cases.



One response to “Toxicology versus solid drug dose examination for controlled substances”